Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Clustered Regularly Interspaced Short Palindromic Repeat-Caspase System: An Approach with Ability for Crop Improvement
*Corresponding author: Nwawuba Stanley Udogadi, Department of Plant Biology and Biotechnology, University of Benin, Nigeria.
Received: May 13, 2022; Published: May 27, 2022
DOI: 10.34297/AJBSR.2022.16.002222
Abstract
Global recent developments and extensive body of evidence have established the fact that crop productivity and yield has declined, and the agricultural sector suffers a huge threat as a result of abiotic and biotic factors. In an attempt to mitigate the challenge of food shortfall, poor plant yield, intolerance to abiotic and biotic stresses and poor adaptability of crops, several methods have been adopted including conventional breeding technologies but has hit a plateau in recent times. However, breakthrough in molecular biology and biotechnology has been demonstrated to provide an improved alternative to the conventional methods for crop improvements. At the present, sequence-specific genome editing technologies particularly the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein9 (Caspase 9) genome editing technology (CRISPR/Cas9) has so far shown the greatest potential in mitigating the emerging challenges in crop improvement. CRISPR/Cas9 technology have been used for specific genome modification in many crops and the progress in CRISPR/Cas9 technology in crop improvement has been outstanding including development of abiotic stress tolerant crop plants, development of disease resistant variety of crop plants, and generation of transgene free genome edited crop plants. There is an expectation that the application of CRISPR/Cas9 technology in variety of crop would revolutionize the agricultural sector in the second green revolution to ensure food and nutritional security of the teeming global population particularly among tropical regions. Therefore, this review provides knowledge on the potentials of CRISPR/Cas9 for crop improvement.
Keywords: CRISPR/Cas9; Crop; Abiotic; Biotic Factors; Palindromic; Ribonucleoprotein; Enzymes; Hybridization; Transgene; Genome Editing; Biotechnologistp>
Introduction
A huge issue in recent times prominent in the world community’s centers around climate change, population growth, food shortage and food insecurity [1]. It has been reported that world population growth is on a rapid increase, and it is projected that by 2050, the population growth would reach 9.7 billion [2,3]. To this backdrop, over 50% population growth expected between now and 2050 are in the tropical region which includes Nigeria, India, Ethiopia, Indonesia, Democratic Republic of the Congo, United Republic of Tanzania and Uganda been the seven countries among the nine with the estimated growth. Particularly, Nigeria is projected to be largest country in standings of population growth after India and China by 2050 [1,3]. The reality of a rapid population growth remains a critical issue and it is worsened by the decrease in availability of arable land joined with a decline in crop yield as a result of climate change and insecurity. According to the International Rice Research Institute (IRRI), it is estimated that in every 7.7s, one hectare of cultivable land is lost and may heighten with the global climate change [1,4]. To match the rapidity in population growth, it has become very necessary that food production and crop yield needs to be increased by 50% by 2030 and 70-100% by 2050 to sustain the world population [5,6].
In an attempt to mitigate the challenge of food shortfall, poor plant yield, intolerance to abiotic and biotic stresses and poor adaptability of crops, several methods have been adopted including conventional breeding technologies but has hit a plateau in recent times [1,7]. However, breakthrough in molecular biology and biotechnology has been demonstrated to provide an improved alternative to the conventional methods for crop improvements [8-10]. Presently, sequence-specific genome editing technologies particularly the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein9 (Caspase 9) genome editing technology (CRISPR/Cas9) has so far shown the greatest potential in mitigating the emerging challenges in crop improvement [11-14]. The CRISPR systems, found in 40% of bacterial and also found in archaea, are a part of natural adaptive immune systems against invading viruses [15,16]. CRISPR-Cas loci on the bacterial genome involves a CRISPR array consisting of up to several hundred direct, often palindromic, repeats (35-45 bases) separated by unique spacer sequences (30-40 bases) [16]. Adjacent to the CRISPR array, is one or more operons having a cluster of Cas genes encoding the effector enzymes of the system [17,18].
CRISPR/Cas systems rely on ribonucleoprotein effector complexes for elimination of invading phages and the immune response provided by the CRISPR-Cas system is categorized into three stages which includes adaptation, pre-CRISPR RNA (crRNA) expression/processing, and interference [19,20]. The ribonucleoprotein effector complex comprises of the nuclease (Cas9) and a guide RNA (gRNA). gRNA specifically binds to the target sequence present in genomic DNA and directs Cas9 to a target site for cleavage, resulting in a double-strand break [21,22]. CRISPR/ Cas system a powerful tool for gene editing finds huge application for targeted genome modification with better precision compared to other conventional genome modification technologies based on the fact that the Cas9 nuclease is guided by RNA rather than proteins which makes CRISPR/Cas9 superior to the transcription activator-like effector nucleases (TALENs) and Zinc-finger nucleases (ZFNs) technologies [15,23]. CRISPR/Cas system has revolutionized medicine, biotechnology, and molecular biology and has attracted extensive attention with its application exponentially growing in the area of plants science since its first application was reported in August 2013 [11,24,25]. The trends of application of the CRISPR/Cas9 technology in mitigating the challenges of crop production has been remarkable. Jia et al. [1,26,27] used CRISPR/ Cas9 to disrupt CsLOB1 gene in grapefruit Duncan (Citrus paradisi Macf.) to produce canker-resistant citrus varieties. CRISPR/Cas9- mediated genome editing was used for the production of transgenefree crop plants; cucumber rice wheat and tomato [28-32]. CRISPR/ Cas9 was also used to develop abiotic stress tolerant crops through the regulatory mechanism of stress/ABA-activated protein kinase2 (SAPK2)-mediated stress tolerance in rice [11,33]. Greatly, this review provides knowledge on the potentials of CRISPR/Cas9 for crop improvement.
Crispr-Cas System
Nakata and colleagues first reported the CRISPR-cas system in 1987 during their study of iap gene. In the study, a set of 29 nucleotide (nt) repeats in E. coli was found however sequencing of genomes of several microbes in the next decade led to the discovery of additional repeat elements in different strains of bacterial and archaeal and this unique repeat element called inter-spaced repeat sequences were then regarded as clustered repeat elements [34- 36]. According to the report of Hsu et al. [37] the term CRISPR was first used in 2002 by Mojica and Jansen. The CRISPR-cas system is used by bacteria and archaea as an adaptive immune system against invaders as well as bacteriophages and Mobile Genetic Elements (MGEs) [38]. The adaptive immune system of the CRISPRCas stores the invaders memory particularly the Mobile Genetic Element (MGE) in the unique spacer derived from MGE and inserts into the CRISPR arrays. The CRISPR spacers transcripts aids the recognition of analogous sequences and direct the Cas nucleases to inactivate targeted familiar MGEs [39,40].
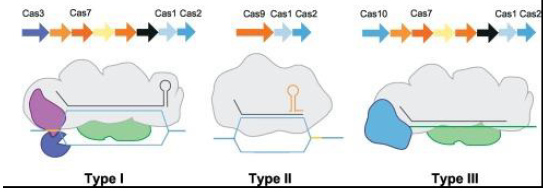
According to the report of Shabbir et al. [34] based on evolutionary examination of Cas proteins, CRISPR/Cas systems as well as signature proteins, Makarova and his colleagues in 2011 suggested a unified nomenclature of CRISPR/Cas systems into three main types, I, II and III [34,41]. On the basis of signature protein, type I has Cas 3, type II has Cas 9 and type type III has Cas 10 as represented in Figure 1. Notably, each class relies on the signature protein to complete its immune response and all the classes have the cas1 and cas2 proteins which plays a key role in the spacer [42- 44]. In terms of complexity, Type I and III makes of use of a multiple signature proteins while Type II makes use of a single protein signature (Cas 9) for producing crRNA and cutting the target DNA and it is also relatively simple to construct and easily engineered to serve as a tool for genome editing [11,41].
Clearly, CRISPR/ Cas9 system has greatly impacted the agricultural sector, however some factors have been identified to limit the efficiency of CRISPR/Cas9 system for editing targeted genes in crop plants which includes the expression levels of sgRNA and cas9, the secondary structure of sgRNAs, the target DNA, and the codons of cas9 and GC content of the target DNA [45,46]. To improve the efficiency, researchers have been able to introduce sgRNA and cas9 expression cassettes into target crop plants via Agrobacterium-mediated transformation and the cas9 gene has also been optimized with plant-usage bias codons [11,19,39,40].
CRISPR-Cas9 Biology and Mechanism
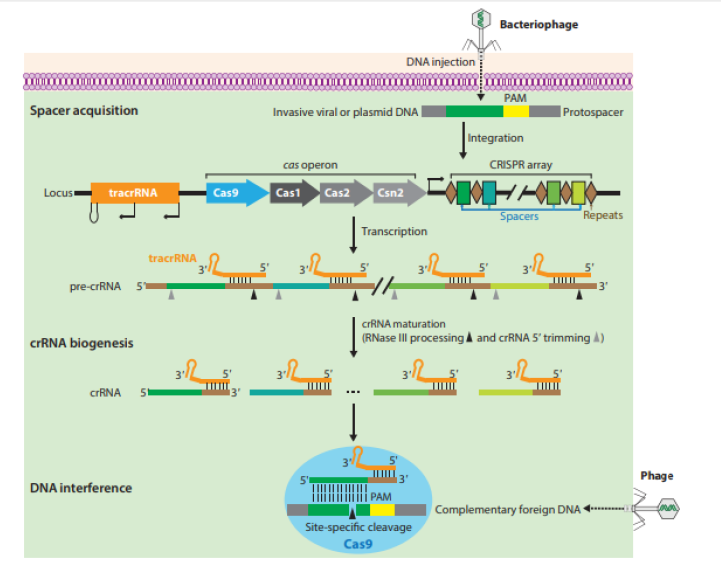
At the time of the discovery of CRISPR the function and mechanism were not yet known [11]. However, a study conducted by Gameau and colleagues in 2010 using Streptococcus thermophilus revealed that CRISPR/Cas was able to cleave the double-stranded DNA of a bacteriophage and plasmid, and these findings demonstrated the molecular basis of adaptive immunity mediated by the CRISPR/Cas system [11,47]. Going further, the key breakthrough that led to the elucidation of the function and mechanism of action of CRISPR system was when it was found out that the spacers within CRISPRs were derived from invading viruses and plasmids [35,48,49]. To provide immunity against invaders, bacteria’s and archaea’s developed RNA-guided adaptive immune systems encoded by CRISPR loci and the associated caspase enzyme [50,51]. The adaptive immune system cascade of event following an invasion from either bacteriophage or mobile genetic elements results to an integration of the foreign DNA materials into CRISPR repeat-spacer array within the host chromosome in form of a new spacers, and as a result offers a genetic record that enables the host to avert subsequent invasion by the similar invader [9,52].
A key feature of CRISPR-Cas systems is the assembly of mature crRNAs from subsequent transcription of the CRISPR array and enzymatic processing of precursor-CRISPR transcripts via Cas protein end nucleolytic cleavage into crRNA-effector complexes to interrogate DNA targets and destroy similar sequences of foreign genetic materials [9,53-55]. Structurally, the crRNA carries the spacer at the 5 end which is an RNA short fragment complimenting the sequence of a foreign genetic element (protospacer) and carries a piece of repeat sequence of the CRISPR at the 3 ends [9,43,56]. Hybridization between the crRNA spacer and protospacer elicits a sequence-specific end nucleolytic damage of foreign genetic materials by Cas nucleases upon subsequent invasion [54,57]. In the process of adaptive immunity of bacteria and archaea via CRISPRCas systems, a short-conserved sequence motif (2-5bp) found close to the crRNA-targeted sequence on the invading DNA, known as the Proto-Spacer Adjacent Motifs (PAMs), plays a necessary part in target DNA selection and degradation [9,11,53]. Mechanistic and morphological studies on of Cas9 activation upon guide RNA binding and target DNA recognition have shown that there is a conformational change for caspase 9 enzyme from an inactive state to an active state (a DNA recognition-competent conformation) upon binding of the guide RNA [9,10,58].
In the DNA recognition-competent conformational state, the RNA seed sequence is preordered in an A-form conformation for target binding and strand invasion, which allows the PAMrecognition sites to preposition for PAM interrogation [10,59,60]. The case 9 enzyme initial binding to PAM sequences results to an interrogation of adjacent DNA for possible target sequence by Cas 9 enzyme [9,56,61]. Following Cas9 finding of possible target sequence with the suitable PAM, a duplex unwinding is initiated, and the sampling of other target sequence continues [43,56]. Finally, for the HNH to achieve a stable and active conformational state for cutting the target strand, there must be a complete annealing of the guide RNA and the target sequence DNA [58]. The resultant change in conformation of the HNH instantaneously results to a great change in conformation of the loop linkers, and in turn direct the nontarget strand to the Revco catalytic center for concerted cleavage [9,56]. After the cleavage, Cas9 enzyme remains firmly bound to the cleaved target DNA sequence till other cellular influences dislodges the enzyme for reprocessing [9,56]. The activity of CRISPR-Cas system consists of three stages: adaptation, expression/processing, and interference (Figure 2).
In the adaptation or spacer acquisition stage, the CRISPRCas loci express a complex of Cas protein which binds to the DNA target sequence and then elicit a two double-strand breaks based on the Protospacer Adjacent Motif (PAM) [19,41,62]. The released DNA target sequence (protospacer) following recognition is then integrated into the CRISPR array which act as a new spacer [19,62,63]. In the expression, processing or biogenesis stage, mature crRNA is generated via the cleavage of pre-crRNA formed by the transcription of CRISPR array by RNA polymerase (RNAP) [64,65]. The cleavage of pre-crRNA to crRNA is catalyzed by specific endoribonucleases and the resultant crRNA is also called the guide RNA based on its function [16,66]. The last stage of the CRISPRCas system activity is the interference. In this stage, the crRNAs recognize and pairs specifically to the foreign sequence of RNA or DNA with nearly perfect complementarity resulting to crRNAforeign nucleic acid complex [41,42]. This results in the cleavage of the crRNA-foreign nucleic acid complex. The mature crRNA, which is bounded to the complex, acts as a guide RNA to recognize similar DNA or RNA sequences in the invading viral RNA that is then cleaved and inactivated by one of the Cas proteins [19,67,68]. However, a cleavage does not occur when there is a mismatch between the spacer and the invader’s DNA [34,69].
Genome Editing Applications Of CRISPR/Cas for Crop Improvement
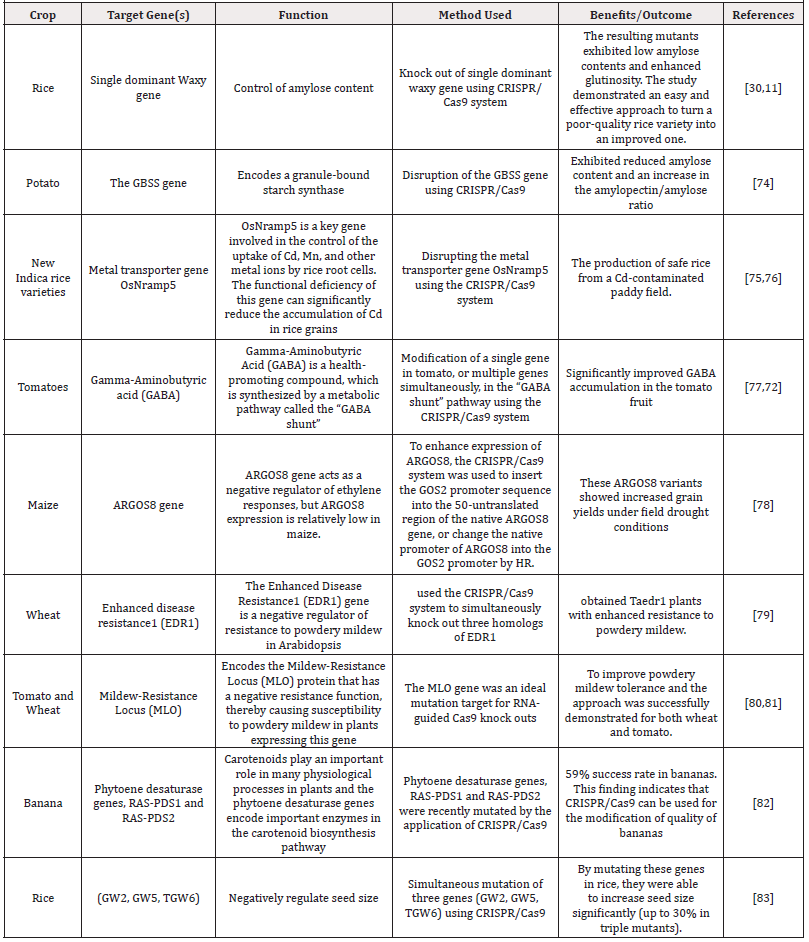
Recent developments worldwide and extensive body evidence have established the fact that crop productivity has declined, and the agricultural sector suffers huge threats as a result of abiotic factors including heat, drought, frost, salinity and biotic factors comprising of fungi, bacterial and viruses as well as insecurity that have displaced rural farmers in the tropical regions of the world [11,30,70]. To this backdrop, the focus of crop breeders is to grow crop with better yield, stress tolerance and disease resistant. CRISPR/Cas9-mediated genome editing does not only find application in functional genomics research, but also provides an innovative way for crop improvement to satisfy the yearnings of crop breeders and meet the growing demand for food [11,30]. The specificity of genome editing provided by CRISPR/Cas9 system have shown huge potentials in crop improvement and emerging evidence have demonstrated a rapid growth of interest in using CRISPR/Cas9 system in the field of agriculture to meet the global growing demand for food [1,24,25,71]. In the last 8 years, the application of CRISPR/Cas9 system for crop improvement have been revealed to be remarkable [1,26,72], and this emerging technology provides an avenue for biotechnologist to develop a productive crop breeding systems with improved yield, abiotic/ biotic stress tolerance and enhanced disease resistance [1,73]. Both presently and in the future, CRISPR/Cas9 system finds huge application and possesses the potential for great value to a large part of crop breeding as presented in Table 1.
Progress of CRISPR/Cas9 Technology in Crop Improvement
This section highlights the application of CRISPR/Cas9 technology in crop plant improvement. Before now, the conventional methods have been used for crop plants genome modification for improved yield, but the technique has now become outmoded as a result of the experienced limitations including requirement for longer time for genome modification, challenges of incompatibility of crop plants species and decreasing plants genetic variations [1,71,73]. In the light of this, crop improvement requires genome modification technology to add novel features to crop plants within the shortest possible time and CRISPR/Cas9 system with its specific transcriptional regulation and genomic modification have extensively been shown to be a suitable alternative for this purpose [74-76]. CRISPR/Cas9 technology have been used for specific genome modification in many crops and the progress in CRISPR/ Cas9 technology in crop improvement has been outstanding including development of abiotic stress tolerant crop plants [77,78], development of disease resistant variety of crop plants [79,80], and generation of transgene free genome edited crop plants [29,79, 81].
The use of crispr/cas9 technology for the development of abiotic stress tolerant crop plants
One of the huge threats to the crop productivity that impedes a worldwide crop yield, is abiotic stress [1, 82]. Some of the key abiotic stresses that have been indicated to affect crop yield includes waterlogging, drought, flooding, temperature, mineral toxicity, soil salinity/acidity and nutrient deficiency and it have widely been reported that these stresses are expected to be aggravated by climate change and environmental degradation [19,83-85]. Abiotic stresses pose a persistent threat to worldwide food security and has resulted to over 40% loss in crop production [84]. To ensure a sustained crop productivity to meet the growing world population despite the climate change, it is now necessary to develop crop plants that can withstand abiotic stresses [85,86]. Molecular studies using CRISPR technology in elucidation of stress responsive genes in plants have identified novel traits and associated genes for the genetic improvement of crop plants [19,84,87]. The phytohormone ethylene have been identified to influence responses of crop plants to abiotic stresses using CRISPR technology [1,83,88].
Similarly, CRISPR/Cas9 system in the study of Wang et al. [89] was used in tomatoes to demonstrate the role of the mitogenactivated protein kinases3 (SlMAPK3) gene in defense responses against drought via analysing the slmapk3 mutants. The study of Lou et al. [33] revealed that the regulatory mechanism of stress/ ABA-activated protein kinase2 (SAPK2)-mediated stress tolerance in rice using a mutant developed using the CRISPR/Cas9 system. To improve grain yield under drought conditions and produce drought-tolerant maize, the CRISPR/Cas9 system was used to insert the GOS2 promoter sequence into the 50-untranslated region of the native ARGOS8 gene [11,90]. CRISPR/Cas9 system has great potential for genetic modification of crop plants to provide tolerance to numerous abiotic factors, increase yield and improve the nutritional quality of crop plants [1,25,91,92].
Crispr/cas9 genome editing for mitigating the challenges of crop diseases and pest
Plant diseases and pests are significant factors affecting crop plant productivity and yield, and a huge challenge of the scientific community is the improvement and application of models for plant diseases to examine and predict the productivity and yield losses including those as result of climate change [93-96]. The impact of diseases and pest on agricultural system have been immense, and several diseases have been identified including brown streak, mosaic and bacterial blight that have caused a significant loss of crop plant yield and productivity [29,30,81,97]. In attempt to mitigate the huge challenges of yield losses loss and productivity of crop plants, a technology that have shown great prospect has become very necessary. In this light CRISPR/Cas9 system have shown great promise and has been used for the development of variety of resistant crop plants against diseases and pest ensuing to improved crop yield and productivity [11,70,79]. Odipio et al. [80] used CRISPR/Cas9 to edit Phyteone desaturase gene in cassava for improved yield. Zaidi et al. [70] utilized CRISPR/Cas9 system for disruption of viral genome to combat plant diseases. The canker susceptibility gene CsLOB1 in grapefruit (Citrus paradisi Macf.) was disrupted to produce a variety of canker-resistant citrus using CRISPR/Cas9 system [27]. Finally, the eukaryotic translation initiation factor 4E (eIF4E) and eukaryotic translation initiation factor isoform 4E (eIF(iso)4E) genes which are identified to be a resistance gene in a wide range of hosts were altered to develop a virus-resistant cucumber (Cucumis sativus) and Arabidopsis plants using the CRISPR/Cas9 system [28,98].
Generation of transgene-free genome edited crops
The simplicity, precision and efficiency of the CRISPR/Cas9 system have been established for genome editing, but its capability for the production of transgene-free genetically modified crops plants has drawn significant interest in recent times [11,29,31]. It has been reported that the transgene-free genetically modified crops plants developed using CRISPR/Cas9 system might bypass the strict biosafety regulations required for genetically modified crops owing to the fact that it is hard to differentiate the transgene-free crop plants from the varieties containing genetic variation created by natural mutagenesis [11,99]. The anti-browning mushroom (Agaricus bisporus) and waxy corn have been reported to already have passed the US biosafety regulations [11]. Additionally, transgene-free genetically modified crops plants developed using CRISPR/Cas9 system have been reported for tomato, rice, maize, wheat, and cucumber [28,30,32, 81, 100,101].
Future Perspective and Conclusion
Global recent developments and extensive body of evidence have established the fact that crop productivity and yield has declined, and the agricultural sector suffers a huge threat as a result of abiotic factors including heat, drought, frost, salinity and biotic factors comprising of fungi, bacterial and viruses. To this backdrop, a switch to a proven technology has become very necessary. The trends of application of the CRISPR/Cas9 technology in mitigating the challenges of crop production has been remarkable. CRISPR/Cas system has revolutionized plant biotechnology and the specificity of genome editing provided by CRISPR/Cas9 system have shown huge potentials in crop improvement and emerging evidence have demonstrated a rapid growth of interest in using CRISPR/ Cas9 system in the field of agriculture to meet the global growing demand for food. Conclusively, the CRISPR/Cas9 system is relatively well understood, its construction methods have progressively been improved, and attempts are ensued to minimize off-target effects as this emerging technology provides an avenue for biotechnologist to develop a productive crop breeding systems with improved yield, abiotic stress tolerance and enhanced disease resistance.
There is an expectation that the application of CRISPR/Cas9 technology in variety of crop would revolutionize the agricultural sector in the second green revolution to ensure food and nutritional security of the teeming global population particularly among tropical regions. Therefore, it is necessary to consider the CRISPR/Cas9 mediated genome crop plants as non-GMO for rapid application and also accept the CRISPR/Cas9 technology at the field level. Additionally, advances in CRISPR/Cas technology for the modification of genes are becoming more applicable for genetic improvement of abiotic stress tolerance in crop plants, and emerging omics approaches provides huge opportunity for elucidation of abiotic stress responses from the cellular to the molecular level of crop plants. In an attempt to improve crop productivity and yield, the scientific community is still faced with the challenges of analysing and predicting crop yield losses due pest and disease including those due to climate change. Models for crop diseases and pest have been shown to predominantly target the applications of pesticides, and as such a holistic approach covering process-based agricultural simulation modelling on one hand, and molecular technology particularly CRISPR/Cas system on the other hand looks promising.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Acknowledgement
None
References
- Haque E, Taniguchi H, Hassan MM, Bhowmik P, Karim MR, et al. (2018) Application of CRISPR/Cas9 Genome Editing Technology for the Improvement of Crops Cultivated in Tropical Climates: Recent Progress, Prospects and Challenges. Front Plant Sci 9: 1-17.
- Clarke JL, Zhang P (2013) Plant biotechnology for food security and bioeconomy. Plant Mol Biol 83(1-2): 1-3.
- Campos H, Caligari PDS (2017) Genetic Improvement of Tropical Crops. Cham: Springer International Publishing.
- Stamm P, Ramamoorthy R, Kumar PP (2011) Feeding the extra billions: strategies to improve crops and enhance future food security. Plant Biotechnol Rep 5: 107-120.
- Godfray HC, Beddington JR, Crute IR, Haddad L, Lawrence D, et al. (2010) Food security: the challenge of feeding 9 billion people. Science 327(5967): 812-818.
- Jones JD, Witek K, Verweij W, Jupe F, Cooke D, et al. (2014) Elevating crop disease resistance with cloned genes. Philos Tran. R Soc Lond B Biol. Sci 369(1639): 20130087.
- Ansari A, Wang C, Wang J, Wang F, Liu P, et al. (2017) Engineered dwarf male-sterile rice: a promising genetic tool for facilitating recurrent selection in rice. Front Plant Sci 8: 2132.
- Nwawuba SU, Mohammed KA, Adams TB, Omusi PI, Ayevbuomwan DE (2020) Forensic DNA Profiling: Autosomal Short Tandem Repeat as a Prominent Marker in Crime Investigation. Malays J Med Sci 27(4): 22-35.
- Jiang F, Doudna JA (2017). CRISPR-Cas9 Structures and Mechanisms. Annu Rev Biophys 46: 505-529.
- Jiang F, Zhou K, Ma L, Gressel S, Doudna JA (2015) Structural Biology. A Cas9-guide RNA complex preorganized for target DNA recognition. Science 348(6242): 1477-1481.
- Aili B, David JB, Haifeng C, Xinan Z, Dong C, et al. (2019) The CRISPR/Cas9 system and its applications in crop genome editing. Crit Rev Biotechnol 39(3): 321-336.
- Georges F, Ray H (2017) Genome editing of crops: a renewed opportunity for food security. GM Crops Food 8(1):1-12.
- Osakabe K, Osakabe Y, Toki S (2010) Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proc Natl Acad Sci USA 107(26): 12034-12039.
- Kumar V, Jain M (2015) The CRISPR-Cas system for plant genome editing: advances and opportunities. J Exp Bot 66(1): 47-57.
- Tawsif AK, Swadesh RB (2021) CRISPR/dCas system as the modulator of gene expression. Prog Mol Biol Transl Sci 178: 99-122.
- Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJH, et al. (2008) Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321(5891): 960-964.
- Makarova KS, Wolf YI, Koonin EV (2013) The basic building blocks and evolution of CRISPR-CAS systems. Biochem Soc Trans 41(6): 1392-1400.
- Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, et al. (2015) An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol 13(11): 722-736.
- Kumar J, Sen GD, Djalovic I, Kumar S, Siddique KHM (2020) Root-omics for drought tolerance in cool-season grain legumes. Physiol Plant 172: 629.
- Komor AC, Badran AH, Liu DR (2017) CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell 169(3): 559.
- Ajit KR, Neeraj KS (2021) Importance of targeted therapies in acute myeloid leukemia. Transl Biotech 5: 107-133.
- Kwang-Hyun P, Yan A, Eui Jeon W (2019) In vitro assembly of thermostable Csm complex in CRISPR-Cas type III/A system. Meth. Enzymol 616: 173-189.
- Li JF, Norville JE, Aach J, McCormack M, Zhang D, et al. (2013) Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol 31(8): 688-691.
- Shan Q, Wang Y, Li J, Gao C (2014) Genome editing in rice and wheat using the CRISPR/Cas system. Nat Protoc 9(10): 2395-2410.
- Abdelrahman M, Al Sadi AM, Pour Aboughadareh A, Burritt DJ, Lam Son PT (2018) Genome editing using CRISPR/Cas9-targeted mutagenesis: an opportunity for yield improvements of crop plants grown under environmental stresses. Plant Physiol Biochem 131: 31-36.
- Rodríguez Leal D, Lemmon ZH, Man J, Bartlett ME, Lippman ZB (2017) Engineering quantitative trait variation for crop improvement by genome editing. Cell 171(2): 470-480.
- Jia H, Zhang Y, Orbovic V, Xu J, White FF, et al. (2017) Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol J 15(7): 817-823.
- Chandrasekaran J, Brumin M, Wolf D, Leibman D, Klap C, et al. (2016) Development of broad virus resistance in nontransgenic cucumber using CRISPR/Cas9 technology. Mol Plant Pathol 17(7): 1140-1153.
- Macovei A, Sevilla NR, Cantos C, Jonson GB, Slamet-Loedin I, et al. (2018) Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol J 16(11): 1918-1927.
- Zhang J, Zhang H, Botella JR, Jian Kang Z (2018) Generation of new glutinous rice by CRISPR/Cas9-targeted mutagenesis of the Waxy gene in elite rice varieties. J Integr Plant Biol 60(5): 369-375.
- Liang Z, Chen K, Zhang Y, Liu J, Yin K, et al. (2018) Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nat Protoc 13(3): 413-430.
- Nekrasov V, Wang C, Win J, Lanz C, Weigel D, et al. (2017) Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci Rep 7(1): 482.
- Lou D, Wang H, Liang G, Yu D (2017) OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Front Plant Sci 8: 993.
- Shabbir MAB, Shabbir MZ, Wu Q, Mahmood S, Sajid A, et al. (2019) CRISPR-cas system: biological function in microbes and its use to treat antimicrobial resistant pathogens. Ann Clin Microbiol Antimicrob 18(1): 21.
- Schindele P, Wolter F, Puchta H (2018) Transforming plant biology and breeding with CRISPR/Cas9, Cas12 and Cas13. FEBS Lett 592(12): 1954-1967.
- Mojica FJM, Díez-Villaseñor C, Soria E, Juez G (2000) Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol Microbiol 36(1): 244-246.
- Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157(6): 1262-78.
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, et al. (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315(5819): 1709-1712.
- Koonin EV, Makarova KS (2019) Origins and evolution of CRISPR-Cas systems. Philos Trans R Soc Lond B Biol Sci 374(17772): 20180087.
- Koonin EV, Makarova KS, Zhang F (2017) Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin. Microbiol 37: 67-78.
- Makarova KS, Haft DH, Barrangou R, Brouns SJJ, Charpentier E, et al. (2011) Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 9(6): 467-477.
- Shabbir MAB, Hao H, Shabbir MZ, Wu Q, Sattar A, et al. (2016) Bacteria vs. bacteriophages: parallel evolution of immune arsenals. Front Microbiol 7: 1-8.
- Rutkauskas MA, Krivoy MD, Szczelkun C, Rouillon RS (2017) Single-Molecule Insight into Target Recognition by CRISPR-Cas Complexes. Meth Enzymol 582: 239-273.
- Kang YK, Kwon K, Ryu JS, Lee HN, Park C, et al. (2017) Nonviral genome editing based on a polymer-derivatized CRISPR nanocomplex for targeting bacterial pathogens and antibiotic resistance. Bioconjug Chem 28(4): 957-967.
- Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, et al. (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8(8): 1274-1284.
- Liu H, Ding Y, Zhou Y, Jin W, Xie K, et al. (2017) CRISPR-P 2.0: an improved CRISPR-Cas9 tool for genome editing in plants. Mol Plant 10(3): 530-532.
- Garneau JE, Dupuis ME, Villion M, Romero DA, Barrangou R, et al. (2010) The CRISPR/cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468(7320): 67-71.
- Francisco J M Mojica, César Díez Villaseñor, Jesús García Martínez, Elena Soria (2005) Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol 60(2): 174-182.
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, et al. (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315(5819): 1709-1712.
- Marraffini LA (2015) CRISPR-Cas immunity in prokaryotes. Nature 526(7571): 55-61.
- Heler R, Marraffini LA, Bikard D (2014) Adapting to new threats: the generation of memory by CRISPRCas immune systems. Mol Microbiol 93(1): 1-9.
- Amitai G, Sorek R (2016) CRISPR-Cas adaptation: insights into the mechanism of action. Nat Rev Microbiol 14(2): 67-76.
- Van der Oost J, Westra ER, Jackson RN, Wiedenheft B (2014) Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nat. Rev. Microbiol 12(7): 479-492.
- Jiang F, Doudna JA (2015) The structural biology of CRISPR-Cas systems. Curr Opin Struct Biol 30: 100-111.
- Wiedenheft B, Sternberg SH, Doudna JA (2012) RNA-guided genetic silencing systems in bacteria and archaea. Nature 482(7385): 331-338.
- Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA (2014) DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 507(7490): 62-67.
- Garneau JE, Dupuis ME, Villion M, Romero DA, Barrangou R, et al. (2010) The CRISPR/cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468(7320): 67-71.
- Palermo G, Miao Y, Walker RC, Jinek M, McCammon JA (2016) Striking plasticity of CRISPR-Cas9 and key role of non-target DNA, as revealed by molecular simulations. ACS Cent Sci 2(10): 756-763.
- Josephs EA, Kocak DD, Fitzgibbon CJ, McMenemy J, Gersbach CA, Marszalek PE (2015) Structure and specificity of the RNA-guided endonuclease Cas9 during DNA interrogation, target binding and cleavage. Nucleic Acids Res 43(18): 8924-8941.
- Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, et al. (2014) Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156(5): 935-949.
- Singh D, Sternberg SH, Fei J, Doudna JA, Ha T (2016) Real-time observation of DNA recognition and rejection by the RNA-guided endonuclease Cas9. Nat Commun 7: 12778.
- Amitai G, Sorek R (2017) Intracellular signaling in CRISPR-Cas defense. Science 357(6351): 550-551.
- Jackson RN, van Erp PB, Sternberg SH, Wiedenheft B (2017) Conformational regulation of CRISPR-associated nucleases. Curr Opin Microbiol 37: 110-119.
- Charpentier E, Richter H, van der Oost J, White MF (2015) Biogenesis pathways of RNA guides in archaeal and bacterial CRISPR-Cas adaptive immunity. FEMS Microbiol Rev 39(3): 428-441.
- Hochstrasser ML, Doudna JA (2015) Cutting it close: CRISPR-associated endoribonuclease structure and function. Trends Biochem. Sci 40(1): 58-66.
- Carte J, Wang R, Li H, Terns RM, Terns MP (2008) Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev 22(24): 3489-3496.
- Plagens A, Richter H, Charpentier E, Randau L (2015). DNA and RNA interference mechanisms by CRISPR-Cas surveillance complexes. FEMS Microbiol Rev 39(3): 442-463.
- Nishiyama J (2019) Genome editing in the mammalian brain using the CRISPR-Cas system. Neurosci. Res 141: 4-12.
- Bhaya D, Davison M, Barrangou R (2011) CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu Rev Genet 45: 273-297.
- Zaidi SS A, Tashkandi M, Mansoor S, Magdy MM (2016) Engineering plant immunity: using CRISPR/Cas9 to generate virus resistance. Front Plant Sci 7: 1673.
- Zhang L, Zhou Q (2014) CRISPR/Cas technology: a revolutionary approach for genome engineering. Sci China Life Sci 57(6): 639-640.
- Li R, Li R, Li X, Fu D, Zhu B, et al. (2018) Multiplexed CRISPR/Cas9-mediated metabolic engineering of c-aminobutyric acid levels in Solanum lycopersicum. Plant Biotechnol J 16(2): 415-427.
- Chen K, Gao C (2014) Targeted genome modification technologies and their applications in crop improvements. Plant Cell Rep 33(4): 575-583.
- Kawakami EM, Oosterhuis DM, Snider JL (2010) Physiological effects of 1-methylcyclopropene on well-watered and water-stressed cotton plants. J. Plant Growth Regul 29: 280-288.
- Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, et al. (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol 32(9): 947-951.
- Kaur N, Alok AS, Kaur N, Pandey P, Awasthi P, et al. (2018) CRISPR/Cas9-mediated efficient editing in Phytoene Desaturase (PDS) demonstrates precise manipulation in banana cv. rasthali genome Funct Integr Genomics 18(1): 89-99.
- Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA (2007) Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci 172: 1113-1123.
- Bart RS, Taylor NJ (2017) New opportunities and challenges to engineer disease resistance in cassava, a staple food of African small-holder farmers. PLoS Pathogen 13(5): 1006287.
- Odipio J, Alieai T, Ingelbrecht I, Nusinow DA, Bart R, et al. (2017) Efficient CRISPR/Cas9 genome editing of phyteone desaturase in cassava. Front Plant Sci 8: 1780.
- Rane J, Singh AK, Kumar M, Boraiah KM, Meena KK, et al. (2021) The Adaptation and Tolerance of Major Cereals and Legumes to Important Abiotic Stresses. Int J Mol Sci 22(23): 12970.
- Tang L, Mao B, Li Y, Qiming L, Zhang L, et al. (2017) Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd accumulating indica rice without compromising yield. Sci Rep 7(1): 14438.
- Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA (2007) Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci 172: 1113-1123.
- Bart RS, Taylor NJ (2017) New opportunities and challenges to engineer disease resistance in cassava, a staple food of African small-holder farmers. Plos Pathogen 13(5): e1006287.
- Rani A, Devi P, Jha UC, Sharma KD, Siddique KHM, et al. (2020) Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. In Front Plant Sci 10: 1759.
- Bailey Serres J, Parker JE, Ainsworth EA, Oldroyd GED, Schroeder JI (2019) Genetic strategies for improving crop yields. Nat 575(7781): 109-118.
- Priya M, Siddique KHM, Dhankher OP, Prasad PVV, Humnath RB, et al. (2018) Molecular breeding approaches involving physiological and reproductive traits for heat tolerance in food crops. Indian J Plant Physiol 23: 697-720.
- Wang L, Chen L, Li R, Zhao R, Yang M, et al. (2017) Reduced drought tolerance by CRISPR/Cas9-mediated SlMAPK3 mutagenesis in tomato plants. J Agric Food Chem 65(39): 8674-8682.
- Xu R, Yang Y, Qin R, Li H, Qiu C, et al. (2016) Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genomics 43(8): 529-532.
- Ou W, Mao X, Huang C, Tie W, Yan Y, et al. (2018) Genome-wide identification and expression analysis of the KUP family under abiotic stress in cassava (Manihot esculenta Crantz). Front Physiol 9: 17.
- Shi J, Gao H, Wang H, Lafitte HR, Rayeann LA, et al. (2017) ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J 15(2): 207-216.
- Ye J, Yang H, Shi H, Wei Y, Tie W, et al. (2017) The MAPKKK gene family in cassava: genome-wide identification and expression analysis against drought stress. Sci Rep 7(1): 14939.
- Liu J, Wang X (2021) Plant diseases and pests detection based on deep learning: a review. Plant methods 17(1): 22.
- Donatelli M, Magarey RD, Bregaglio S, Willocquet L, Whish J, et al. (2017) Modelling the impacts of pests and diseases on agricultural systems. Agricul Sys 155: 213-224.
- Bregaglio S, Frasso N, Pagani V, Stella T, Francone C, et al. (2015) New multi-model approach gives good estimations of wheat yield under semi-arid climate in Morocco. Agron. Sustain Dev 35: 157-167.
- Bregaglio S, Titone P, Cappelli G, Tamborini L, Mongiano G, et al. (2016) Coupling a generic disease model to the WARM rice simulator to assess leaf and panicle blast impacts in temperate climate. Eur J Agron 76: 107-117.
- Pyott DE, Sheehan E, Molnar A (2016) Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol Plant Pathol 17(8): 1276-1288.
- Tuteja N, Verma S, Sahoo RK, Raveendar S, Reddy BL (2012) Recent advances in development of marker-free transgenic plants: regulation and biosafety concern. J Biosci 37(1): 167-197.
- Tuteja N, Verma S, Sahoo RK, Raveendar S, Reddy BL (2012) Recent advances in development of marker-free transgenic plants: regulation and biosafety concern. J Biosci 37(1): 167-197.
- Svitashev S, Schwartz C, Lenderts B, Young JK, Cigan AM (2016) Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat Commun 7: 13274.
- Zhang Y, Liang Z, Zong Y, Wang Y, Liu J, et al. (2016) Efficient and transgene- free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat Commun 7: 12617.
- Andersson M, Turesson H, Nicolia A, Ann Sofie F, Samuelsson M (2017) Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep 36(1): 117-128.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.